39 cautionary and advisory labels for medicines
bnf.nice.org.uk › about › labelsCautionary and advisory labels | About | BNF | NICE To be used on preparations containing ofloxacin and some other quinolones, doxycycline, lymecycline, minocycline, and penicillamine. These drugs chelate calcium, iron, and zinc and are less well absorbed when taken with calcium-containing antacids or preparations containing iron or zinc. bnf.nice.org.uk › about › guidance-for-cautionaryGuidance for cautionary and advisory labels | About | BNF | NICE Wordings which can be given as separate warnings are labels 1–19, 29–30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21–28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted.
bnfc.nice.org.uk › drugs › macrogol-3350-withMedicinal forms | Macrogol 3350 with potassium chloride ... There can be variation in the licensing of different medicines containing the same drug. Oral solution. All products. Show Cautionary and advisory labels. Label 13 . Dissolve or mix with water before taking. Gadewch i doddi mewn dŵr cyn ei gymryd. Electrolytes. May contain bicarbonate, chloride, potassium, sodium. Show
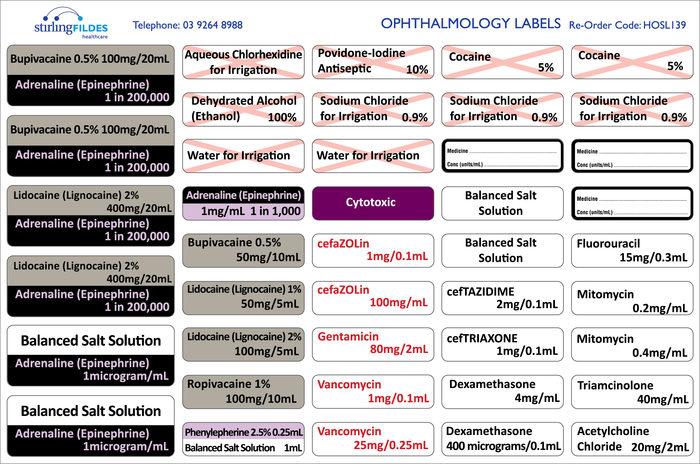
Cautionary and advisory labels for medicines
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ... Cautionary and advisory labels for dispensed medicines Cautionary and advisory labels for dispensed medicines Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary. Cautionary Advisory Labels Flashcards | Quizlet Cautionary Advisory Labels. STUDY. PLAY. Diazepam. o Label 1: This medicine may cause DROWSINESSS and may increase effects of alcohol. If affected, do not drive a motor vehicle or operate machinery o Label 1a: This preparation is to aid sleep. Drowsiness may continue the following day. If affected, do not drive or operate machinery.
Cautionary and advisory labels for medicines. Medicinal forms | Sterculia | Drugs | BNFC | NICE Cautionary and advisory labels. Label 25 . Swallow this medicine whole. Do not chew or crush. Llyncwch yn gyfan. Peidiwch â chnoi neu falu'n fân. Label 27 . Take with a full glass of water. Cymerwch gyda llond gwydr o ddŵr. Show Normacol granules Forum Health Products Ltd Show Cautionary and advisory labels. Label 25 . Swallow this ... Guidance for cautionary and advisory labels For BNF for Children 2011‒2012, a revised set of cautionary and advisory labels were introduced. All of the existing labels were user-tested, and the revised wording selected reflects terminology that is better understood by patients. ... Pharmacists label medicines with various wordings in addition to those directions specified on the ... Labels on medicines and poisons - Department of Health Labels on medicines and poisons All medicines and poisons containers must be labelled so as to clearly identify the contents. This is important to prevent inadvertent consumption and poisoning. Labels must meet uniform Australian standards on text size, language and warnings. Labels on poisons Dispensing labels Labels on medicines More information Medicinal forms | Fenfluramine | Drugs | BNFC | NICE There can be variation in the licensing of different medicines containing the same drug. Oral solution All products Show Cautionary and advisory labels Excipients May contain hydroxybenzoates (parabens), glucose. Show Sugar free Fintepla 2.2mg/ml oral solution Zogenix International Ltd Back to top
Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness 50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning. bnfc.nice.org.uk › aboutAbout | BNFC | NICE Free online access to the UK BNFC (British National Formulary for Children) content published by NICE Medicinal forms | Tapentadol | Drugs | BNFC | NICE Oral solution There can be variation in the licensing of different medicines containing the same drug. Oral solution All products Show Cautionary and advisory labels Excipients May contain propylene glycol. Show CD2 Sugar free Palexia 20mg/ml oral solution Grunenthal Ltd Back to top
› consumers › consumer-updatesConsumer Updates | FDA Jun 02, 2022 · Consumer Updates Science-based health and safety information you can trust. Pharmacy Cautionary and Advisory Labels Cautionary and Advisory Labels - Cautionary Advisory Labels (CALs) are essential consumer resources that promote quality use of medicines. Showing 1-9 of 114 results Cautionary & Advisory Label Holder $ 50.80 Compare; cautionary & advisory label no. 139 $ 9.50 Compare; cautionary & advisory label no. 140 ... Cautionary Advisory Labels Flashcards | Quizlet Cautionary Advisory Labels. STUDY. PLAY. Diazepam. o Label 1: This medicine may cause DROWSINESSS and may increase effects of alcohol. If affected, do not drive a motor vehicle or operate machinery o Label 1a: This preparation is to aid sleep. Drowsiness may continue the following day. If affected, do not drive or operate machinery. Cautionary and advisory labels for dispensed medicines Cautionary and advisory labels for dispensed medicines Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary.
› drugs › drug-safety-and-availabilityFDA Drug Safety Communication FDA Briefing Information for the February 10-11, 2014 Joint Meeting of the Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee. Available from:. Accessed December ...
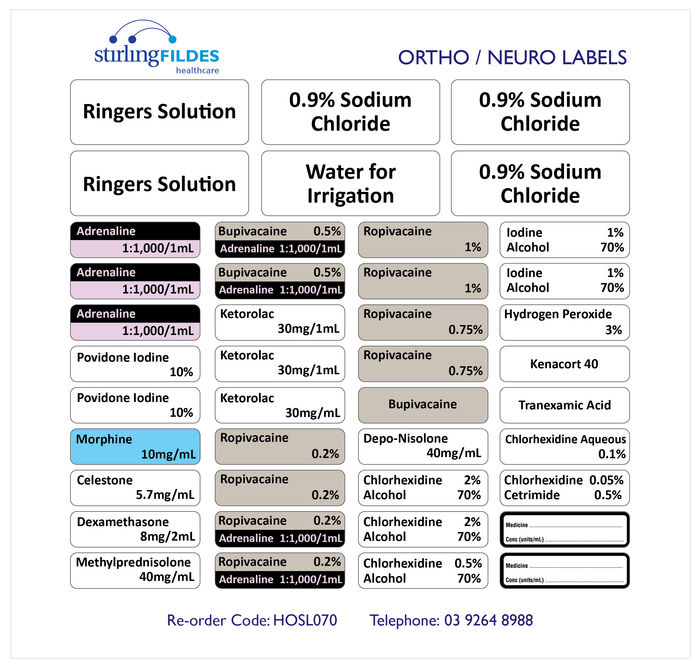



Post a Comment for "39 cautionary and advisory labels for medicines"